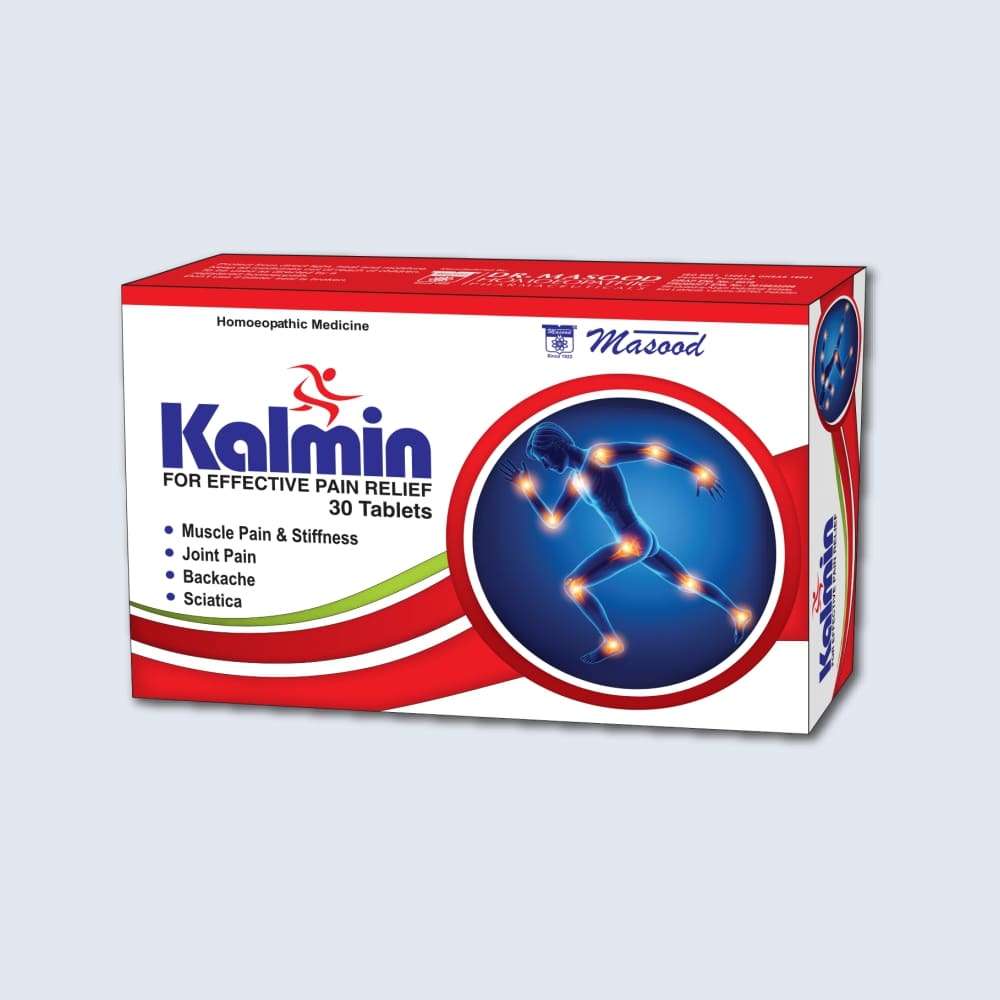
KALMIN (Effective Pain Relief) 30 Tablets
₨ 340 Original price was: ₨ 340.₨ 310Current price is: ₨ 310.
26 in stock
Description
Masood’s Kalmin tablets is an ideal Homoeopathic pain reliever (pain killer) for all kinds of body pains.
- Karmin tablets are Indicated for joint & muscular pains
- Kalim is an effective homoeopathic pain relief remedy for toothache.
- Effective in reducing headache, lumbago and sciatica.
- Used for renal colic, post-traumatic pains.
- Also helpful in treating the acute attacks of gout.
Dosage Directions for use
- Rheumatoid arthritis, Ankylosing spondylitis and osteoarthritis: 2-3 Tablets three times a day.
- Lumbago, sciatica: 2 Tablets four times a day, in Acute conditions, 2 Tablets three times a day when improvement begins.
- Bursitis, Tendinitis, Myositis: 2 Tablets two times a day.
- Acute attack of Gout: 2 Tablets every four hours.
- Renal colic: 2-3 Tablets three times a day.
- Dysmenorrhoea: 2 Tablets three times a day.
- Dental pain: 2 Tablets three times a day.
- Post-operative and Post traumatic Inflammation: 2-3 Tablets four times a day.
Warnings:
- Keep all medicines out of reach of children.
- Keep in a cool and dry place.
- To be used as directed by a registered homeopath.
- Use within three years of manufacturing.
- Do not use if blister seal is broken.
Contraindications:
Kalmin may not be used in patients having peptic ulcer and urticaria.
Reviews (0)
Be the first to review “KALMIN (Effective Pain Relief) 30 Tablets” Cancel reply
Shipping & Delivery


📦 Shipping Information
We ensure safe, timely delivery of your homeopathic medicines to preserve their efficacy. Here’s what you need to know:
🚚 Domestic Shipping
Standard Delivery: 3–5 business days.
⚡ Important Notes:
Homeopathic medicines are shipped in secure, temperature-safe packaging.
Dispatch time: 24–48 hours (excluding Sundays/holidays).
Remote areas: May incur extra delivery time.
You may also like…
CINERARIA EYE DROPS (Cataract Retainer)(10ml)
₨ 165
CINERARIA MARITIMA (Excellent Nourishment for Eyes)
₨ 310
DERMI CLEAR (For Skin Cleanser and a Powerful Antiseptic)
Related products
Ornitho + H.Pylori Drops for Stomach (Indigestion, Heart Burn, Flatulence and Gastritis)
₨ 650
Rated 5.00 out of 5
Dr. Reckeweg R 190 Digestion Drops for Stomach
₨ 2,230
Dr. Reckeweg R 5 Stomach Drops
₨ 1,170
ALSHOKA Syrup Kamal (Female Hormones Optimizer)
₨ 230
DYRO Syrup (For relief in all types of diarrhoea)
₨ 200
KUFZIT (CAPSULES) (Relieves cough and cold) (20 capsules)
₨ 200
KL 4 (SYZOPHOS) (Don’t get mad, get relief diabetes) (20ml)
₨ 160










Reviews
There are no reviews yet.